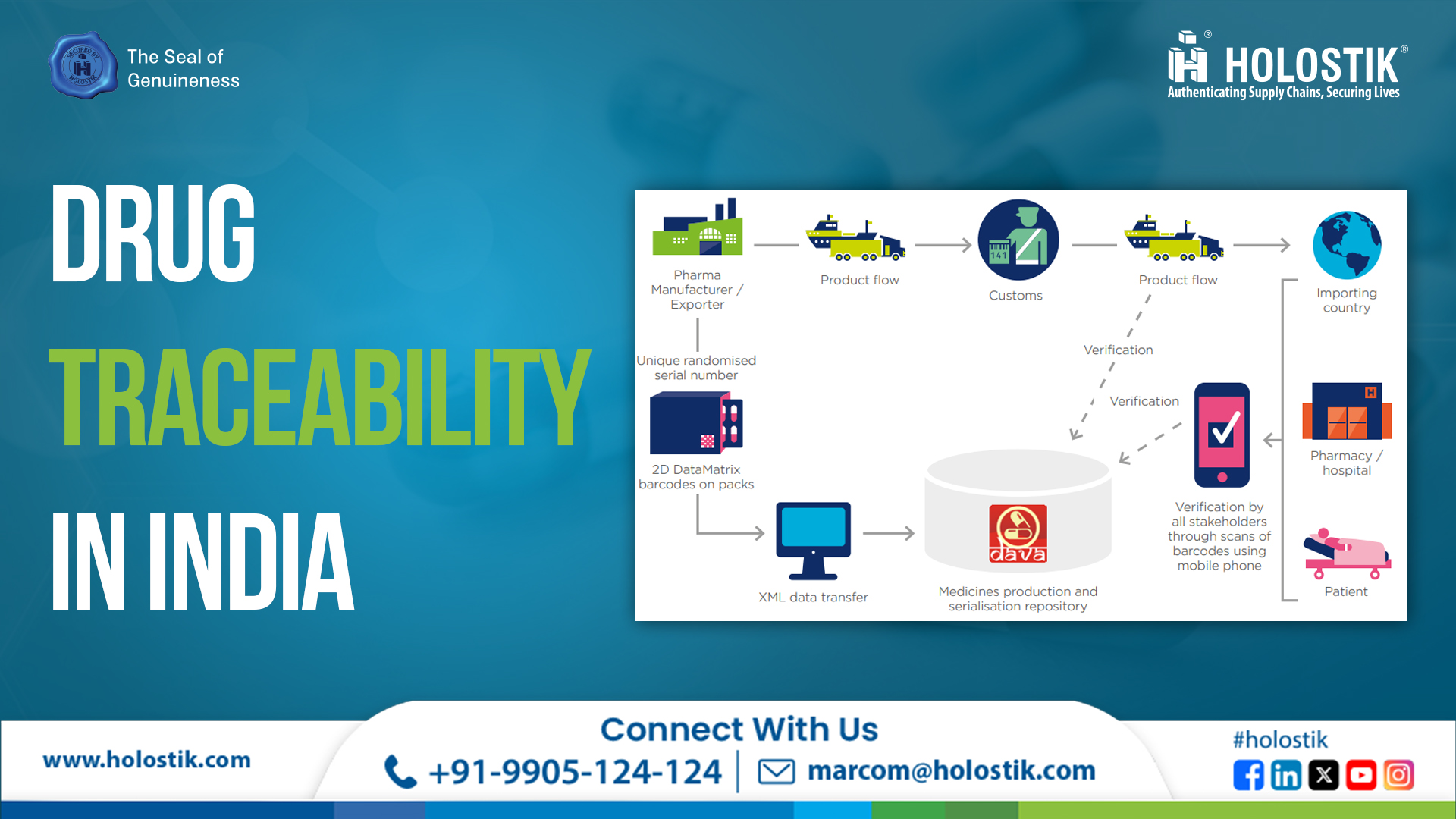
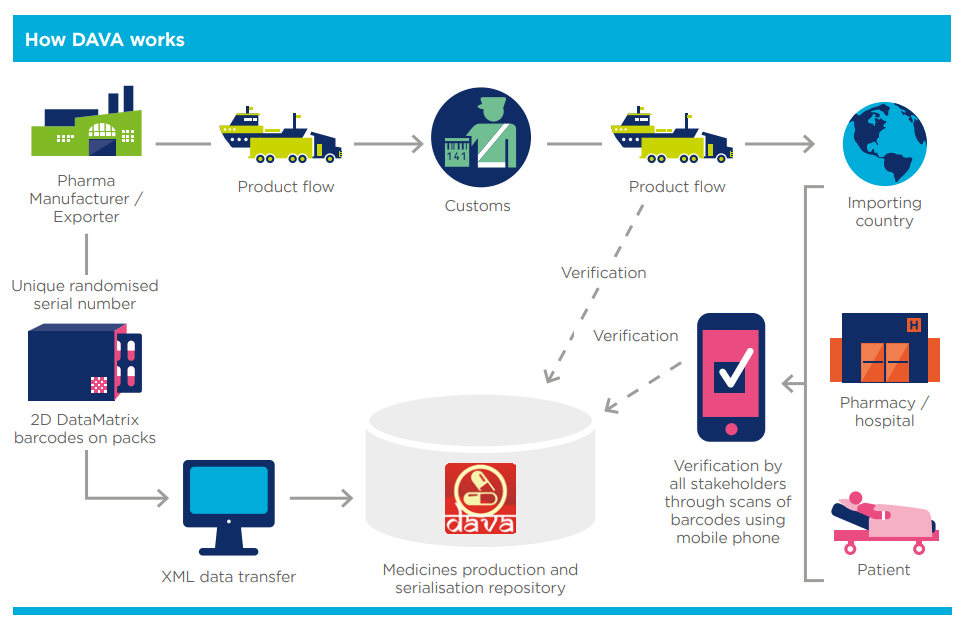
More than a decade ago, India’s Commerce Ministry launched a national-level drug traceability programme called DAVA — the Drug Authentication and Verification Application.
It was designed to curb the export of counterfeit medicines, especially those headed to African markets.
But after years of delays, confusion, and compliance chaos, the government withdrew the mandate earlier this year.
So, what went wrong? And more importantly, what comes next for India’s fight against fake medicines?
At Holostik, we’ve spent over 30 years building solutions that secure brands and save human lives.
The lessons from this failed experiment revealed the urgent need for practical, integrated anti-counterfeiting systems designed for India’s real-world supply chains.
Where the Traceability Model Broke Down
When the system launched in 2011, pharma companies rushed to comply with new serialization and aggregation requirements. Dozens of machine and software vendors appeared overnight claiming to offer “end-to-end traceability.”
But most lacked the deep understanding of data complexity, aggregation logic, and multi-node integration required for such a massive ecosystem.
The so-called “Traceability Bottleneck” soon emerged at the stockist-to-pharmacy level.
With 65,000+ stockists and over half a million pharmacies in India, re-aggregation became practically impossible.
The system collapsed under its own logistical weight. Industry experts later acknowledged that aggregation and interoperability challenges were the root cause of the failure.
The Human and Economic Cost
While the digital infrastructure faltered, counterfeit drugs thrived.
According to the World Health Organization, substandard and falsified medical products claim over half a million lives each year.
Meanwhile, Indian manufacturers spent crores installing barcode systems that ultimately offered no traceability — only digital redundancy.
As ASPA’s Counterfeiting Report highlights, counterfeit incidents in the Indian pharmaceutical sector continued to rise despite the mandate.
Why Traceability Alone Isn’t Enough
Traceability, while powerful in theory, cannot combat counterfeiting on its own. It’s like tracking a package — but without guaranteeing that what’s inside is genuine.
Counterfeiting networks don’t always operate within traceable supply chains. They exploit weak points like stockists and distributors to inject fake products.
That’s why authentication, the ability to verify a product’s genuineness instantly, must precede traceability.
The Holostik Way: Phygital Traceability That Works
At Holostik, we’ve redefined what traceability truly means — by merging physical authentication and digital intelligence into one seamless, standards-compliant framework.
A. Physical Security That Can’t Be Faked
We integrate optical variable devices (security holograms), tamper-proof films and high-security labels into packaging.
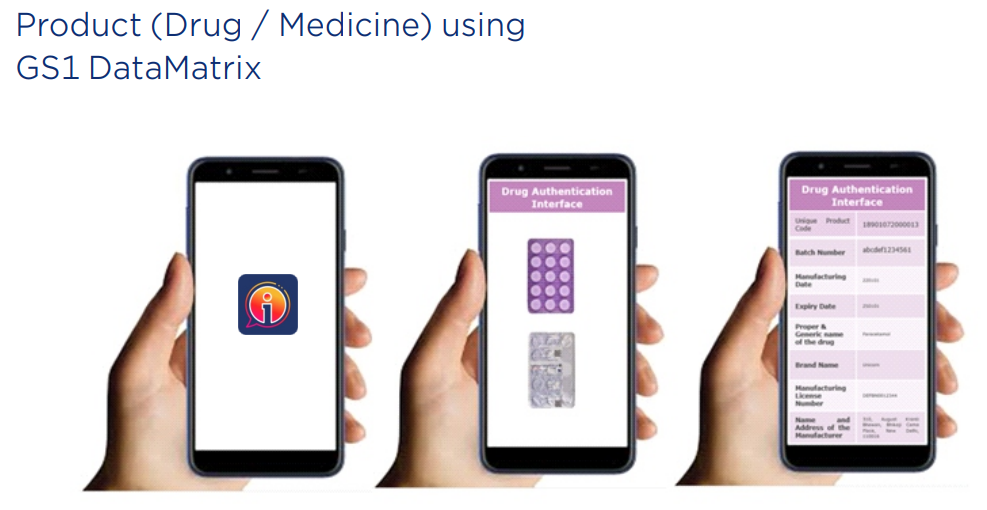
Each pack carries visible and covert features, combined with a unique serialized GS1 DataMatrix code.
Why GS1 DataMatrix matters:
This 2D barcode format encodes product identifiers like GTIN, batch number, expiry, and serial number — enabling interoperability across the global supply chain.
GS1 standards ensure that every pack is universally identifiable and machine-readable, facilitating accuracy and compliance.
B. Digital Intelligence That Connects
Through Utopia Digitech (a Holostik Group company) and its flagship SaaS based solutions SureAssure, brands can implement comprehensive product authentication and traceability systems.
With our digital solutions, brands gain:
- End-to-end serialization & aggregation (compliant with GS1 standards)
- Real-time supply chain visibility
- Consumer-level authentication through GS1 Digital Link URI — allowing users to scan the code with a smartphone and access authenticity details directly
- ERP and CRM integration for unified control and seamless operations
Why GS1 Digital Link URI?
It transforms static barcodes into interactive web links — enabling dynamic, secure consumer engagement, product recalls, and traceability data access.
C. Actionable Data That Protects
Every scan builds intelligence — mapping duplication hotspots, gray market leakages, and sales patterns.
That’s how Holostik helps brands achieve up to 100% visibility and 90% drop in duplication — not theory, but real-world impact.
Benefits of GS1-compliant traceability systems:
- Global interoperability with regulatory systems
- Data accuracy and transparency across nodes
- Faster recalls and better pharmacovigilance
- Improved supply chain efficiency
- Trust and safety for consumers
Rethinking the Future
The collapse of India’s drug traceability programme isn’t the end — it’s an opportunity to rebuild smarter.
The next generation of pharmaceutical security must focus on:
- Authentication before aggregation
- Local adaptability over global templates
- Phygital synergy instead of isolated systems
- GS1-driven interoperability and transparency
At Holostik, we’re already enabling that shift — protecting brands across 90+ countries and multiple industries.
Conclusion
The myth of drug traceability exposed a hard truth:
Without authentication, traceability is just data. Without integration, it’s just theory.
As India charts a new course, Holostik stands ready with turnkey GS1-compliant phygital solutions that secure every pack, every shipment, and every patient.
Because authenticity saves lives — and that’s a promise we’ve kept for over 30 years.