Counterfeit medicines pose a severe threat to global health, with the World Health Organization (WHO) estimating that 10% of medicines in low- and middle-income countries are substandard or falsified. In India, a leading producer of generic drugs, counterfeit pharmaceuticals not only endanger lives but also damage the country’s reputation as a reliable exporter.
To address this challenge, the GS1 Track and Trace system has emerged as a robust solution, enabling end-to-end visibility and authentication of products across the supply chain. In this article, we explore how GS1 standards are revolutionizing pharmaceutical traceability, India’s role in global compliance, and how Holostik, a GS1-certified company, provides advanced digital anti-counterfeiting solutions.

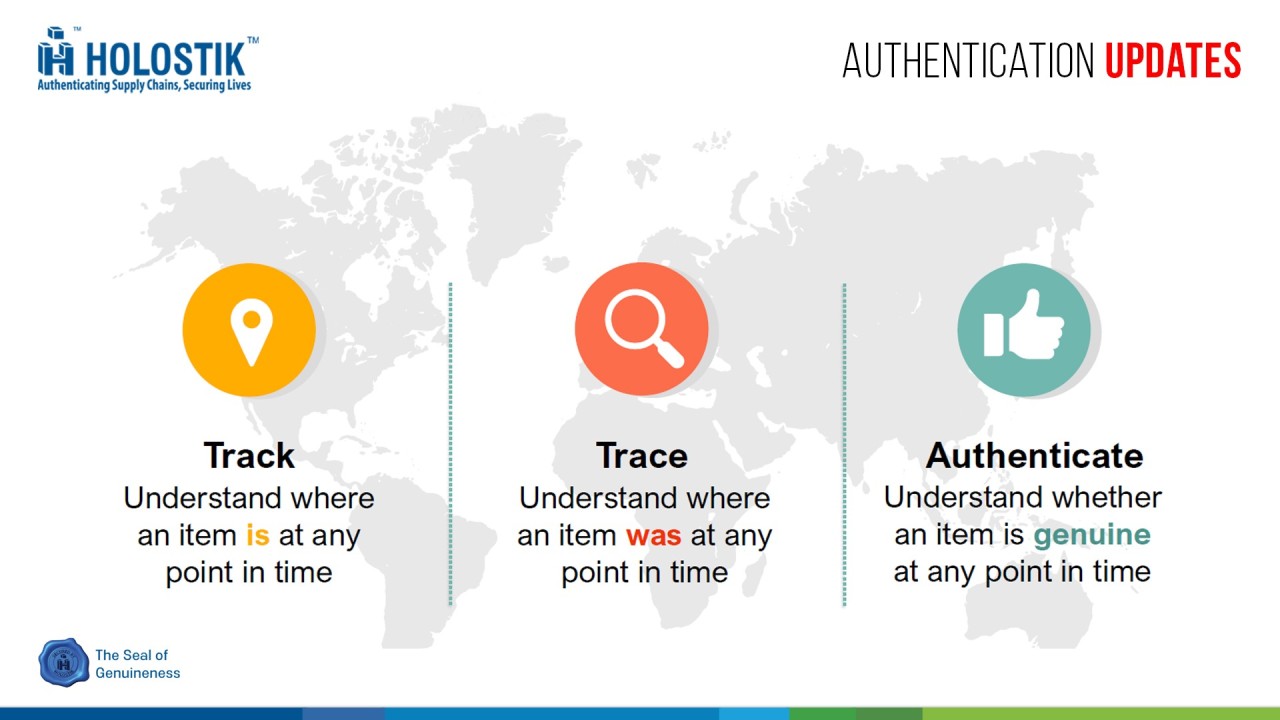
What is GS1 Track and Trace?
The GS1 Track and Trace system is a global standard for product identification, authentication, and traceability. It uses unique barcodes (such as GS1 DataMatrix and GS1-128) to track products from manufacturing to consumption.

Key Components of GS1 Track & Trace
GS1 Identification Keys are globally unique identifiers that help organizations standardize and share information about products, locations, and documents across supply chains. These keys enhance visibility and traceability and require a GS1 Company Prefix obtained through GS1 membership.

How GS1 Track & Trace Works?
1. Serialization: Each product is assigned a unique serial number encoded in a 2D DataMatrix barcode.
2. Aggregation: Products are linked hierarchically (e.g., bottles → cartons → pallets).

3. Authentication: Consumers, regulators, and supply chain stakeholders can verify product authenticity via scanning.

4. Centralized Database: Governments or regulatory bodies maintain a repository for real-time tracking (e.g., India’s Drug Authentication and Verification Application (DAVA)).
Overview of DGFT Requirements on Pharma Packaging
The requirements specify the following use of GS1 standards for coding and marking products at various packaging levels:

Primary Level Packaging
Primary level packaging is defined as the first level of packaging that is in direct contact with the product. This packaging level is marked with an Automatic Identification and Data Capture (AIDC) data carrier (e.g. GS1 DataMatrix) either on the packaging itself or on a label affixed to the packaging. Marking products at this level is optional.

Secondary Level Packaging
Secondary level packaging is defined as a level of packaging that may contain one or more primary packages, or a group of primary packages containing a single item, marked with an AIDC (Automatic Identification and Data Capture) data carrier (e.g., GS1-128, GS1 DataMatrix) either on the packaging or on a label affixed to the packaging.

As per the requirements, monocartons should be treated as secondary level packages. However, affixing a barcode on mono-cartons containing a single item such as a strip, blister pack, bottle or vial is optional at this time.
Tertiary Level Packaging
Tertiary level packaging, for the purpose of this document, is defined as the shipper, carton, or pallet that contains one or more primary/secondary levels of packaging.
India’s Leadership in Pharma Track & Trace
- India is the 3rd largest pharmaceutical producer by volume, supplying 20% of global generics.
- The Drug Authentication and Verification Application (DAVA) system, developed by India’s National Informatics Centre (NIC), ensures real-time tracking of exported medicines.

Key Benefits:
- Faster customs clearance (barcode scanning replaces manual checks).
- Prevents counterfeit drugs from entering the supply chain.
- Enhances India’s credibility as a trusted pharma exporter.

To strengthen the fight against counterfeit drugs, the Government of India has implemented two major traceability initiatives in the pharmaceutical sector – Pharma 300 and API traceability. Under Pharma 300, the top 300 brands in India must carry QR codes on their primary packaging, enabling verification of vital product details by regulators and consumers. Similarly, the API traceability regulation ensures that each Active Pharmaceutical Ingredient used in drug manufacturing is trackable across the supply chain. Both mandates aim to enhance transparency, authenticity, and patient safety.
Each QR code must include the following 11 details:
- Name of the API
- Unique product identification code
- Brand name (if any)
- Batch number
- Manufacturing license number
- Name and address of the manufacturer
- Batch size
- Date of expiry or retesting
- Serial shipping container code
- Date of manufacturing
- Special storage conditions required (if any)
3 Essential Tips for Choosing the Right Label for Your Pharma Product

Holostik: Your Trusted GS1-Certified Anti-Counterfeiting Partner
As a GS1-certified company, Holostik offers end-to-end digital anti-counterfeiting solutions that comply with global track and trace mandates.
Why Choose Holostik?
✔ GS1-Compliant Serialization & Aggregation
- Unique 2D DataMatrix barcodes for product authentication.
- Parent-child hierarchy for seamless supply chain tracking.
✔ Mobile & Web-Based Verification
- Consumers can scan QR Codes via SureAssure mobile app for instant authentication.
- Businesses gain real-time insights into product movement.
✔ Government & Industry Compliance
- Supports DGFT (India), EU FMD, DSCSA (USA), and other global regulations.
✔ End-to-End Security Solutions
- Tamper-evident labels, holograms, and blockchain integration for enhanced security.
Holostik’s Digital Anti-counterfeiting Services:
- GS1-compliant Barcodes and QR Codes: Providing unique digital identities for products, enabling authentication through scanning.
- Track and Trace Technology: Equipping manufacturers with the ability to monitor their products throughout the supply chain, from production to the end consumer.
- Real-time Authentication: Allowing customers to verify the genuineness of products at the point of purchase via scanning QR codes or barcodes.
- Supply Chain Visibility: Enabling manufacturers to track the movement of individual products, reducing the risk of counterfeiting and diversion.
- Integration Capabilities: Offering solutions that can be seamlessly integrated with existing ERP, CRM, and BI systems.
By partnering with Holostik, pharmaceutical companies can leverage GS1 standards and cutting-edge digital technologies to effectively combat counterfeiting, enhance patient safety, and protect their brand reputation. Holostik’s commitment to quality and innovation makes them a valuable partner in the ongoing efforts to secure the pharmaceutical supply chain.

Holostik’s Impact in Numbers

- 41% increase in product sales reported by clients.*
- 90% reduction in product duplication incidents.*
- 339% ROI for brands adopting Holostik’s solutions.*
- 99.9% cloud uptime for uninterrupted service.*
*Numbers are derived from specific case studies.
The Future of Track & Trace
With AI, IoT, RFID and blockchain integration, the next phase of track and trace will enable:

Conclusion
The GS1 Track and Trace system is no longer optional – it’s a regulatory necessity for pharmaceutical companies aiming to combat counterfeiting, ensure patient safety, and maintain global market access.
Holostik’s GS1-certified solutions provide the perfect blend of technology, compliance, and security, making it the ideal partner for brands looking to safeguard their products and reputation.
Secure your supply chain today with Holostik—because every genuine product matters!

Would you like a customized track-and-trace solution for your business?
Reach out now to explore how we can help: 📞 +91 9905-124-124 | ✉ marcom@holostik.com | 🌐 www.holostik.com